Chemistry, 21.07.2019 03:00 breannaasmith1122
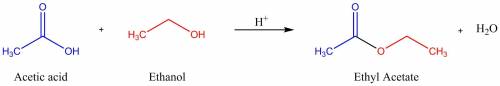
Ethyl acetate is a sweet-smelling solvent used in varnishes and fingernail polish remover. it is produced industrially by heating acetic acid and ethanol together in the presence of sulfuric acid, which is added to speed up the reaction. the ethyl acetate is distilled off as it is formed. the equation for the process is as follows.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

You know the right answer?
Ethyl acetate is a sweet-smelling solvent used in varnishes and fingernail polish remover. it is pro...
Questions

Mathematics, 14.07.2019 08:30


Geography, 14.07.2019 08:30



Mathematics, 14.07.2019 08:30

English, 14.07.2019 08:30

Mathematics, 14.07.2019 08:30

Mathematics, 14.07.2019 08:30

Mathematics, 14.07.2019 08:30


Social Studies, 14.07.2019 08:30


Biology, 14.07.2019 08:30

Physics, 14.07.2019 08:30




History, 14.07.2019 08:30

Mathematics, 14.07.2019 08:30