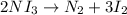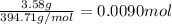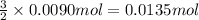Chemistry, 21.07.2019 06:00 itzmelanie1
The chemical equation below shows the decomposition of nitrogen triiodide (ni3) into nitrogen (n2) and iodine (i2). 2ni3 mc030-1.jpg n2 + 3i2 the molar mass of i2 is 253.80 g/mol, and the molar mass of ni3 is 394.71 g/mol. how many moles of i2 will form 3.58 g of ni3?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

You know the right answer?
The chemical equation below shows the decomposition of nitrogen triiodide (ni3) into nitrogen (n2) a...
Questions

Health, 19.11.2019 20:31

Geography, 19.11.2019 20:31




Chemistry, 19.11.2019 20:31


Computers and Technology, 19.11.2019 20:31

History, 19.11.2019 20:31



Health, 19.11.2019 20:31



Mathematics, 19.11.2019 20:31





Mathematics, 19.11.2019 20:31