
Biology, 06.01.2020 12:31 RebelZane18
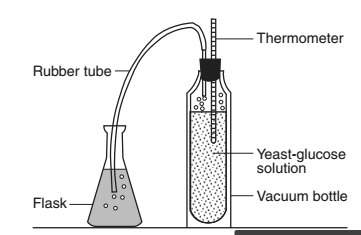
"in the experimental setup below, which substance would be used to prove that the gas produced by the yeast in the vacuum bottle could change the ph of the liquid in the flask?
(1)an indicator
(2)a chemical messenger
(3)an enzyme
(4)a salt solution"

Answers: 2


Another question on Biology

Biology, 21.06.2019 23:30
Which level of consumer had access to the smallest apply of energy
Answers: 1

Biology, 22.06.2019 03:00
Where does all the water go? according to the environmental protection agency (epa), in a typical wetland environment, 39% of the water is outflow; 46% is seepage; 7% evaporates; and 8% remains as water volume in the ecosystem (reference: united states environmental protection agency case studies report 832-r-93-005). chloride compounds as residuals from residential areas are a problem for wetlands. suppose that in a particular wetland environment the following concentrations (mg/l) of chloride compounds were found: outflow, 60.4; seepage, 73.7; remaining due to evaporation, 26.4; in the water volume, 46.8. (a) compute the weighted average of chlorine compound concentration (mg/l) for this ecological system. (round your answer to one decimal place.) mg/l (b) suppose the epa has established an average chlorine compound concentration target of no more than 58 mg/l. does this wetlands system meet the target standard for chlorine compound concentration? yes. the average chlorine compound concentration (mg/l) is too high. yes. the average chlorine compound concentration (mg/l) is lower than the target. no. the average chlorine compound concentration (mg/l) is lower than the target. no. the average chlorine compound concentration (mg/l) is too high.
Answers: 3

Biology, 22.06.2019 10:00
In what part of the body does the most muscle activity happen?
Answers: 1

Biology, 22.06.2019 21:00
Hydrogen has only one electron in its only (and outer) electron shell. if a hydrogen atom were to absorb a small amount of energy, where would bohr say the electron would go? a. fly off the atom b. nowhere, it would just speed up. c. spiral inwards towards the nucleus d. move up to another shell that would form
Answers: 2
You know the right answer?
"in the experimental setup below, which substance would be used to prove that the gas produced by th...
Questions

Mathematics, 12.02.2020 04:35






Biology, 12.02.2020 04:35

History, 12.02.2020 04:35









Computers and Technology, 12.02.2020 04:35





