Biology, 07.12.2019 13:31 fjjjjczar8890
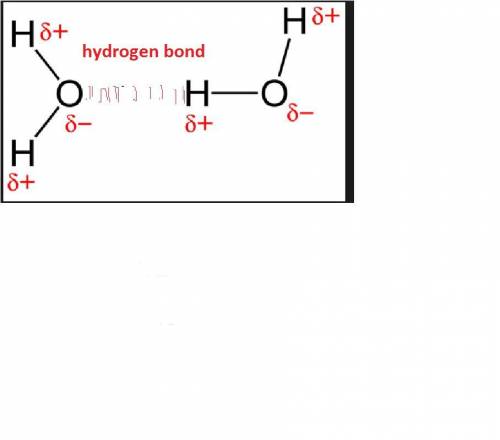
Which of the following best explains how hydrogen bonding affects the heat of vaporization for water?
a. the hydrogen bonds cause water to resist a change in temperature.
b. the number of hydrogen bonds in water increases the amount of h+ ions available.
c. the uneven pull of hydrogen bonds causes water molecules to repel each other.
d. the hydrogen bonds cause water to be highly attracted to other substances.

Answers: 3


Another question on Biology


Biology, 22.06.2019 09:30
What type of plant is good for a bioassay and where can i buy it? i only have a month.
Answers: 2

Biology, 22.06.2019 11:00
Why did adult crab numbers decline in he ecosystem, even though sea stars were still available?
Answers: 2

Biology, 22.06.2019 15:00
Why should organisms reproduce more offspring than will survive? select all that apply.
Answers: 2
You know the right answer?
Which of the following best explains how hydrogen bonding affects the heat of vaporization for water...
Questions

Medicine, 18.11.2019 12:31


Business, 18.11.2019 12:31

Mathematics, 18.11.2019 12:31



Mathematics, 18.11.2019 12:31


Mathematics, 18.11.2019 12:31




History, 18.11.2019 12:31