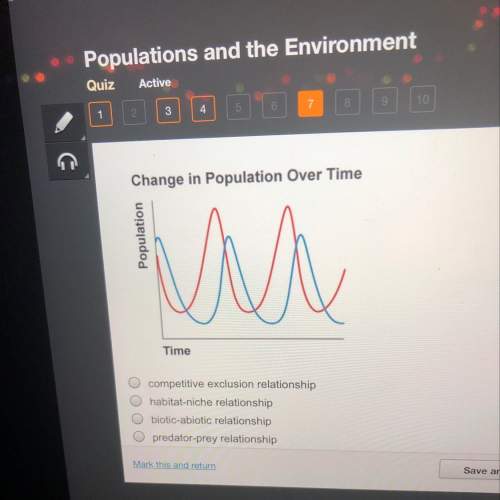
Biology, 18.12.2020 21:50 amoakoh800003
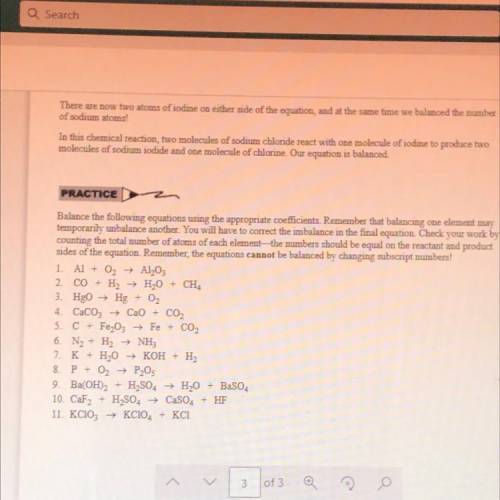
Balance the following equations using the appropriate coefficients. Remember that balancing one element may
temporarily unbalance another. You will have to correct the imbalance in the final equation. Check your work by
counting the total number of atoms of each element—the numbers should be equal on the reactant and product
sides of the equation. Remember, the equations cannot be balanced by changing subscript numbers!
1. Al + O2 → Al,03
2. CO + H2 + H2O + CH4
3. HgO → Hg + O2
4. CaCO3 + CaO + CO2
5. C + Fe,03 → Fe + CO2
6. Na + H2 + NH3
7. K + H2O → KOH + - H2
P + O2 → P203
9. Ba(OH)2 H2SO4 H2O + BaSO4
10. CaF2 + H2SO4 → CaSO4 + HF
KC104 + KCI
8.
-
11. KCIO:
Ok

Answers: 2


Another question on Biology



Biology, 22.06.2019 00:30
Ais a landform that is formed at the mouth of a river from the deposition of sediment carried by the river as the water flows.
Answers: 2

You know the right answer?
Balance the following equations using the appropriate coefficients. Remember that balancing one elem...
Questions

History, 23.01.2020 05:31

Biology, 23.01.2020 05:31

Chemistry, 23.01.2020 05:31



Advanced Placement (AP), 23.01.2020 05:31



Physics, 23.01.2020 05:31







History, 23.01.2020 05:31

Mathematics, 23.01.2020 05:31