
Biology, 21.02.2020 18:36 akornegay2
A certain buffer is made by mixing a weak acid HA and its conjugate base A–. When HA is present at a concentration of 0.5 mM and A– is present at a concentration of 0.1 mM, the buffer has a pH of 6.16. Calculate the ratio of the concentrations of HA and A– when the buffer has a pH of 7.02.

Answers: 3


Another question on Biology


Biology, 22.06.2019 05:00
Will mark brainliest. (20 points) many have pigments structures known as eyespots that detect direction of light. a. b. archaebacteria c. protists d. none of the above
Answers: 1


Biology, 22.06.2019 09:00
Which kind of worm has a closed circulatory system? a planarian b fluke c pinworm d an earthworm
Answers: 1
You know the right answer?
A certain buffer is made by mixing a weak acid HA and its conjugate base A–. When HA is present at a...
Questions

Health, 12.10.2020 14:01

English, 12.10.2020 14:01


Mathematics, 12.10.2020 14:01

English, 12.10.2020 14:01



Chemistry, 12.10.2020 14:01

Mathematics, 12.10.2020 14:01

Mathematics, 12.10.2020 14:01


Mathematics, 12.10.2020 14:01


Mathematics, 12.10.2020 14:01



English, 12.10.2020 14:01



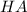 and
and 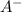 when the buffer has a pH of 7.02 is 0.69
when the buffer has a pH of 7.02 is 0.69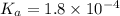
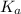 .
.
![pH=pK_a+\log \frac{[A^-}{[HA]}](/tpl/images/0519/3185/d76d0.png)
![6.16=pK_a+\log (\frac{[0.1]}{0.5})](/tpl/images/0519/3185/4ed04.png)
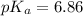
![7.02=6.86+\log \frac{[A^-]}{[HA]}](/tpl/images/0519/3185/a5047.png)
![\frac{[A^-]}{[HA]}=1.44](/tpl/images/0519/3185/0f1f0.png)
![\frac{[HA]}{[A^-]}=0.69](/tpl/images/0519/3185/ef74e.png)


