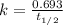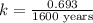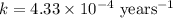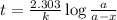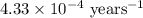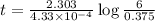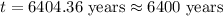The isotope radium-226 decays into radon-222, with a half-life of around 1,600 years. if a rock contained 6 grams of radium-226 when it reached its closure temperature but only 0.375 grams when it was discovered, which two statements about the rock are true? a] the rock reached its closure temperature 6,400 years ago. b] the rock reached its closure temperature 4,800 years ago. c]the rock had 2.625 grams of radon-222 1,600 years ago. d]when the rock was discovered, it had 5.625 grams of radon-222. e]when the rock was discovered, it had 3.375 grams of radon-222.

Answers: 1


Another question on Biology


Biology, 22.06.2019 01:00
What can be said about farmers in highly developed countries? a) they have little or no negative impact on the environment. b) they practice subsistence agriculture. c) they are able to incorporate polyculture into their farming practices. d) they utilize organic farming techniques on a regular basis. e) they rely on large amounts of energy from fossil fuels.
Answers: 3

Biology, 22.06.2019 02:00
Which best describes what you will do in the reaching your academic potential course?
Answers: 2

Biology, 22.06.2019 03:00
Where does all the water go? according to the environmental protection agency (epa), in a typical wetland environment, 39% of the water is outflow; 46% is seepage; 7% evaporates; and 8% remains as water volume in the ecosystem (reference: united states environmental protection agency case studies report 832-r-93-005). chloride compounds as residuals from residential areas are a problem for wetlands. suppose that in a particular wetland environment the following concentrations (mg/l) of chloride compounds were found: outflow, 60.4; seepage, 73.7; remaining due to evaporation, 26.4; in the water volume, 46.8. (a) compute the weighted average of chlorine compound concentration (mg/l) for this ecological system. (round your answer to one decimal place.) mg/l (b) suppose the epa has established an average chlorine compound concentration target of no more than 58 mg/l. does this wetlands system meet the target standard for chlorine compound concentration? yes. the average chlorine compound concentration (mg/l) is too high. yes. the average chlorine compound concentration (mg/l) is lower than the target. no. the average chlorine compound concentration (mg/l) is lower than the target. no. the average chlorine compound concentration (mg/l) is too high.
Answers: 3
You know the right answer?
The isotope radium-226 decays into radon-222, with a half-life of around 1,600 years. if a rock cont...
Questions








Physics, 25.09.2019 00:10





Mathematics, 25.09.2019 00:10


Mathematics, 25.09.2019 00:10

Mathematics, 25.09.2019 00:10



Mathematics, 25.09.2019 00:10

English, 25.09.2019 00:10