
Advanced Placement (AP), 08.04.2021 21:50 manlycool7543
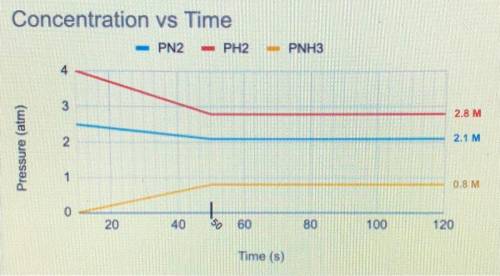
Question 1:
The balanced equation below is a reversible reaction. The concentration of each chemical species was
measured at various time points.
N2(g)+3 H2 (g) <—> 2NH3 (g)
a) When was equilibrium reached?
b) Write the equilibrium expression.
c) Would you expect the Keg to be greater than or less than 1? Justify your answer using data from the graph.

Answers: 2


Another question on Advanced Placement (AP)

Advanced Placement (AP), 22.06.2019 04:00
Which of the following is a disadvantage of using solar energy? a. it requires good sun exposure. b. it is abundantly available. c. it is a renewable resource. d. it reduces greenhouse gas emissions.
Answers: 3

Advanced Placement (AP), 23.06.2019 19:40
Want free points and brainliest? answer this drivers ed question correctly and you shall receive : ) in which of the following situations is it legal to pass? a. the vehicle you want to pass is driving at or above the speed limit in front of you. b. you must cross a center line that's solid on your side and broken on the opposite side. c. you are in the middle of crossing a bridge that only has one lane each direction. d. you are on a straight road and can pass the car ahead under the speed limit.
Answers: 2

Advanced Placement (AP), 25.06.2019 23:00
If you walk up stairs,how u gonna pet yo dog? if you go to colloge,how you gonna fly? if you like school how u gonna be a person?
Answers: 2

Advanced Placement (AP), 26.06.2019 05:30
Treatment of psychological disorders: a. can offer significant relief b. often makes the problem worse. c.is often unclear d. is rarely .
Answers: 2
You know the right answer?
Question 1:
The balanced equation below is a reversible reaction. The concentration of each chemica...
Questions


Mathematics, 23.04.2021 01:00

English, 23.04.2021 01:00

Advanced Placement (AP), 23.04.2021 01:00

Chemistry, 23.04.2021 01:00

English, 23.04.2021 01:00

Law, 23.04.2021 01:00

History, 23.04.2021 01:00




Social Studies, 23.04.2021 01:00


Mathematics, 23.04.2021 01:00









