
Advanced Placement (AP), 10.03.2021 03:50 lnc0500
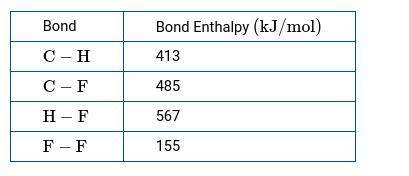
CH4(g) gas reacts with F2(g) to produce CH3F(g) and HF(g). b.) Use the bond enthalpies in the table below to calculate a numerical estimate of ΔH for the reaction.

Answers: 2


Another question on Advanced Placement (AP)

Advanced Placement (AP), 23.06.2019 19:30
On a time limit! willing to give free 25 points plus brainliest for this drivers ed question if answered correctly : ) systematic searching is a skill that takes to master. a. no time at all b. repetitive practice c. innate talent d. extra gadgets
Answers: 2

Advanced Placement (AP), 24.06.2019 11:20
Arectangle is removed from a right triangle to create the shaded region shown below. find the area of the shaded region.be sure to include the correct unit in your answer.14 mmm² mxlal? 4m3m
Answers: 1

Advanced Placement (AP), 25.06.2019 05:30
The probability of rolling a sum of 7 when rolling two dice simultaneously is 0.167. you decide to test that probability by rolling th dice 12 times. what is the probability that exactly 2 of the rolls is a sum of 7?
Answers: 1

Advanced Placement (AP), 26.06.2019 08:30
Which side did jupiter chose in the trojan war
Answers: 1
You know the right answer?
CH4(g) gas reacts with F2(g) to produce CH3F(g) and HF(g).
b.) Use the bond enthalpies in the table...
Questions


Mathematics, 25.02.2020 05:28

Mathematics, 25.02.2020 05:28



















