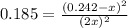Advanced Placement (AP), 26.02.2021 16:40 jdvazquez18p7a7vs
For the equilibrium: 2 NO (g) ⇌ N 2(g)+ O 2(g)at 300 K, the equilibrium constant, Kc, is 0.185. If 1.45 moles each of N 2(g)and O 2(g)are introduced in a container that has a volume of 6.00 liters and allowed to reach equilibrium at 300 K, what are the concentrations of N 2(g ) , O 2(g) ,and NO (g)at equilibrium?

Answers: 1


Another question on Advanced Placement (AP)

Advanced Placement (AP), 23.06.2019 02:30
Daryl wouod like to open new checkings and savings accounts one pf his primary concerns is avoiding bank fees
Answers: 2

Advanced Placement (AP), 23.06.2019 14:50
I’m so stuck on this question i’ll give anybody brainliest and free points for the correct give the most space to a. the largest vehicle b. the fastest road user c. a potential drunk driver d. the biggest hazard
Answers: 2

Advanced Placement (AP), 23.06.2019 21:30
According to binet, mental age relates to chronological age because a. they are the same thing b. mental age involves calculating the chronological age at which a person functions c. chronological age involves calculating how a person is mentally functioning d. they are opposites 38
Answers: 1

You know the right answer?
For the equilibrium: 2 NO (g) ⇌ N 2(g)+ O 2(g)at 300 K, the equilibrium constant, Kc, is 0.18...
Questions

Geography, 26.03.2021 17:50





Mathematics, 26.03.2021 17:50

Mathematics, 26.03.2021 17:50


Mathematics, 26.03.2021 17:50



Mathematics, 26.03.2021 17:50

Mathematics, 26.03.2021 17:50


Mathematics, 26.03.2021 17:50


Mathematics, 26.03.2021 17:50


Mathematics, 26.03.2021 17:50

Biology, 26.03.2021 17:50

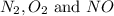 at equilibrium are 0.112 M, 0.112 M and 0.260 M
at equilibrium are 0.112 M, 0.112 M and 0.260 M = 1.45 mole
= 1.45 mole = 1.45 mole
= 1.45 mole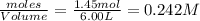
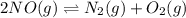
![K_c=\frac{[N_2]\times [O_2]}{[NO]^2}](/tpl/images/1150/8878/68b6f.png)